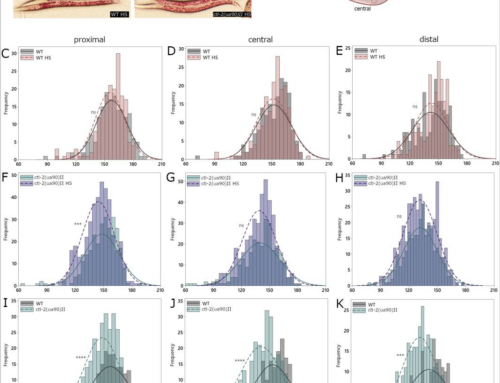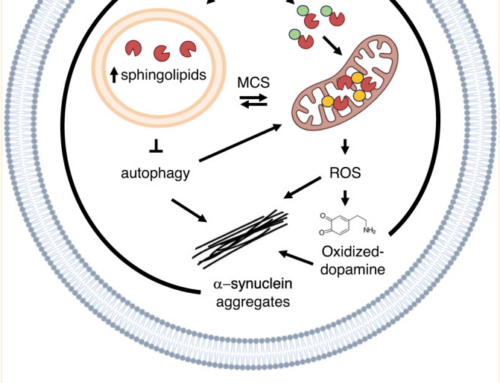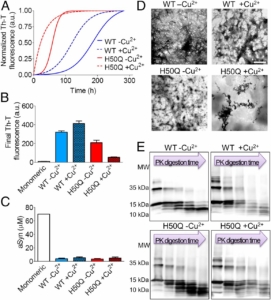
Synucleinopathies are a group of progressive disorders characterized by the abnormal aggregation and accumulation of α-synuclein (aSyn), an abundant neuronal protein that can adopt different conformations and biological properties. Recently, aSyn pathology was shown to spread between neurons in a prion-like manner. Proteins like aSyn that exhibit self-propagating capacity appear to be able to adopt different stable conformational states, known as protein strains, which can be modulated both by environmental and by protein-intrinsic factors. Here, we analyzed these factors and found that the unique combination of the neurodegeneration-related metal copper and the pathological H50Q aSyn mutation induces a significant alteration in the aggregation properties of aSyn. We compared the aggregation of WT and H50Q aSyn with and without copper, and assessed the effects of the resultant protein species when applied to primary neuronal cultures. The presence of copper induces the formation of structurally different and less-damaging aSyn aggregates. Interestingly, these aggregates exhibit a stronger capacity to induce aSyn inclusion formation in recipient cells, which demonstrates that the structural features of aSyn species determine their effect in neuronal cells and supports a lack of correlation between toxicity and inclusion formation. In total, our study provides strong support in favor of the hypothesis that protein aggregation is not a primary cause of cytotoxicity.