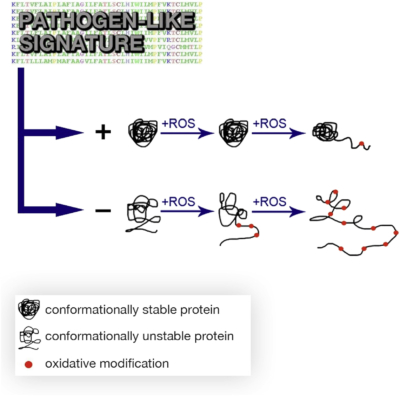
Protein oxidation is known to compromise vital cellular functions. Therefore, invading pathogenic bacteria must resist damage inflicted by host defenses via reactive oxygen species. Using comparative genomics and experimental approaches, we provide multiple lines of evidence that proteins from pathogenic bacteria have acquired resistance to oxidative stress by an increased conformational stability. Representative pathogens exhibited higher survival upon HSP90 inhibition and a less-oxidation-prone proteome. A proteome signature of the 46 pathogenic bacteria encompasses 14 physicochemical features related to increasing protein conformational stability. By purifying ten representative proteins, we demonstrate in vitro that proteins with a pathogen-like signature are more resistant to oxidative stress as a consequence of their increased conformational stability. A compositional signature of the pathogens’ proteomes allowed the design of protein fragments more resilient to both unfolding and carbonylation, validating the relationship between conformational stability and oxidability with implications for synthetic biology and antimicrobial strategies.